Classification of Carbohydrates Carbohydrates can be classified into several categories based on their chemical structure and complexity. Here are the primary classifications of carbohydrates with examples for each:
1. Monosaccharides (Simple Sugars):
- Monosaccharides are the simplest carbohydrates, consisting of a single sugar molecule.
- Examples:
- Glucose: Found in blood and serves as the primary source of energy for cells.
- Fructose: Naturally occurring sugar in fruits and honey.
- Galactose: Found in milk and dairy products.
2. Disaccharides:
- Disaccharides are composed of two monosaccharide molecules linked together.
- Examples:
- Sucrose: Composed of glucose and fructose; found in table sugar and plants.
- Lactose: Composed of glucose and galactose; found in milk and dairy products.
- Maltose: Composed of two glucose molecules; found in malted foods and beverages.
3. Oligosaccharides:
- Oligosaccharides consist of 3 to 10 monosaccharide units linked together.
- Examples:
- Raffinose: Found in beans, broccoli, and other vegetables.
- Stachyose: Present in legumes, seeds, and some vegetables.
4. Polysaccharides (Complex Carbohydrates):
- Polysaccharides are large molecules made up of numerous monosaccharide units linked together.
- Examples:
- Starch: A storage form of glucose in plants, found in foods like potatoes, rice, and bread.
- Glycogen: The storage form of glucose in animals, primarily in the liver and muscles.
- Cellulose: A type of fiber found in plant cell walls, not digestible by humans but important for digestive health.
- Chitin: Found in the exoskeletons of arthropods (e.g., insects) and fungal cell walls.
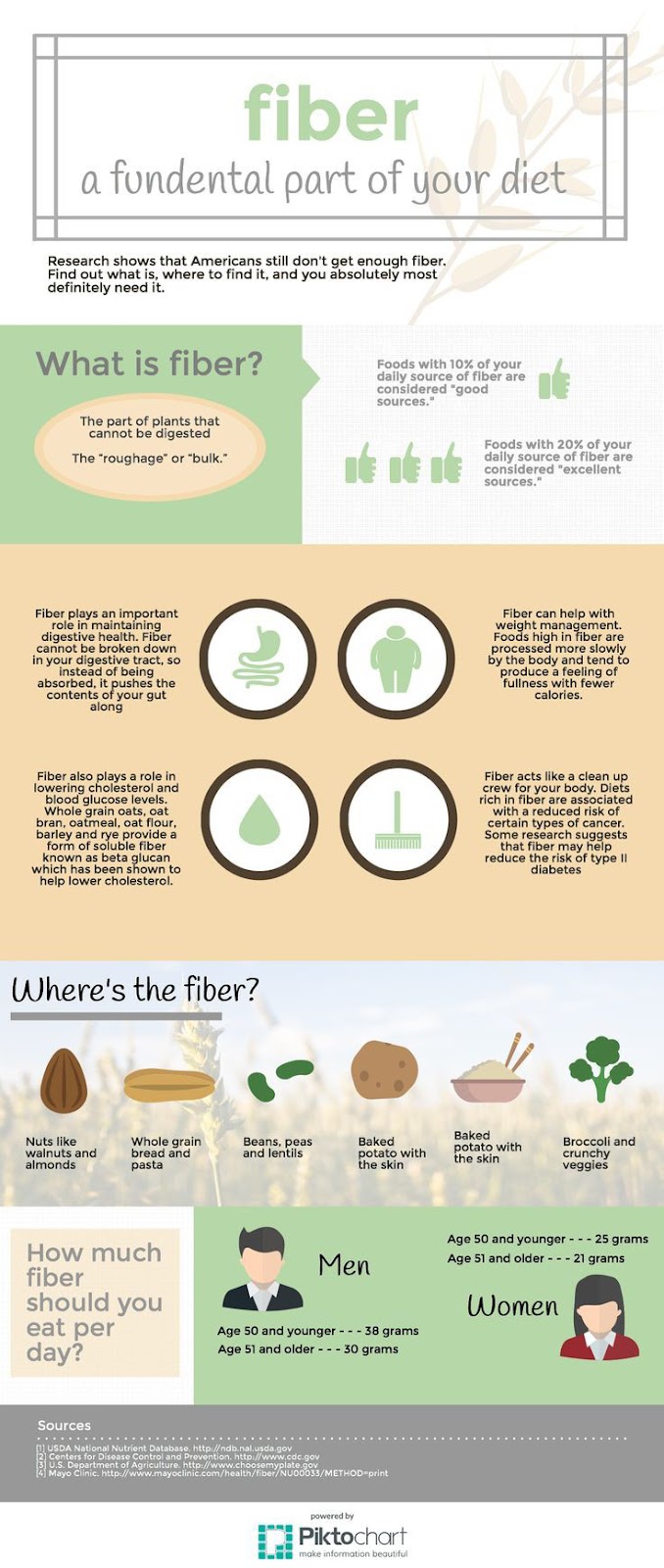
5. Dietary Fiber:
- Dietary fiber includes various indigestible carbohydrates that provide health benefits.
- Examples:
- Soluble Fiber: Found in oats, beans, and fruits; helps lower cholesterol and stabilize blood sugar.
- Insoluble Fiber: Found in wheat bran, vegetables, and whole grains; aids in digestive regularity.
- Resistant Starch: A type of starch that resists digestion in the small intestine, found in undercooked potatoes and green bananas.
These classifications represent the diversity of carbohydrates found in foods, each with its own role in nutrition and health. It's important to include a variety of carbohydrate sources in your diet to ensure a balanced intake of different types of carbohydrates, as they offer different nutritional benefits.
Chemical Tests for Carbohydrates:-
Chemical tests for carbohydrates are used to identify the presence of carbohydrates in a substance. Here are some common chemical tests for carbohydrates:
1. Benedict's Test:
- Benedict's reagent is used to test for reducing sugars, such as glucose and fructose.
- The substance to be tested is mixed with Benedict's reagent and heated in a water bath.
- If reducing sugars are present, the solution changes from blue to green, yellow, orange, or even brick-red, depending on the concentration of reducing sugars.
2. Iodine Test:
- The iodine test is used to detect the presence of starch, which is a complex carbohydrate.
- A few drops of iodine solution (iodine dissolved in potassium iodide) are added to the substance being tested.
- If starch is present, the solution turns from brown to blue-black.
3. Fehling's Test:
- Fehling's solution A (copper sulfate) and Fehling's solution B (sodium potassium tartrate and sodium hydroxide) are mixed in equal proportions.
- This test is primarily used for reducing sugars like glucose.
- The substance is heated with the Fehling's reagent in a water bath.
- If reducing sugars are present, a reddish-orange precipitate of copper(I) oxide forms.
4. Barfoed's Test:
- Barfoed's reagent (copper acetate in acetic acid) is used to differentiate between monosaccharides and disaccharides.
- Monosaccharides, such as glucose, react more quickly and give a red precipitate within a few minutes, while disaccharides, like sucrose, require a longer time.
5. Seliwanoff's Test:
- Seliwanoff's reagent (resorcinol in concentrated HCl) is used to distinguish between aldose and ketose sugars.
- Ketose sugars, such as fructose, react rapidly and produce a cherry-red color, whereas aldose sugars react more slowly and give a faint pink color.
These tests provide qualitative information about the presence of carbohydrates in a sample and can help differentiate between various types of carbohydrates based on their chemical properties.